- The Mass Number Of An Element Is Equal To The
- The Mass Number Of An Element Is Equal To The Number Of Protons And Electrons
The mass number is equal to the: number of protons and neutrons: The atomic number is equal to the number of in an atom: protons: The number below the chemical symbol on the periodic table that has a few decimal places is known as the. Average atomic mass: If an element has a mass # of 23 and an atomic # of 11, how many protons will. The atomic mass of an element is equal to the proton number plus the neutron number What is the atomic mass of an element equal to? Democritus was the first person to suggest the idea of atoms in the fourth century Who was the first person to suggest the idea of atoms in the fourth century? The mass number of an element is equal to the Total number of Neutron and Protons in its nucleolus. Mass number = Number of neutron + number of Proton.
Learning Outcomes
- Define atomic and mass numbers.
- Determine the number of protons, neutrons, and electrons in an atom.
- Identify the charge and relative mass of subatomic particles.
- Label the location of subatomic particles in the atom.
- Determine the mass of an atom based on its subatomic particles.
- Write A/Z and symbol-mass format for an atom.
Atoms are the fundamental building blocks of all matter and are composed of protons, neutrons, and electrons. Because atoms are electrically neutral, the number of positively charged protons must be equal to the number of negatively charged electrons. Since neutrons do not affect the charge, the number of neutrons is not dependent on the number of protons and will vary even among atoms of the same element.

Atomic Number
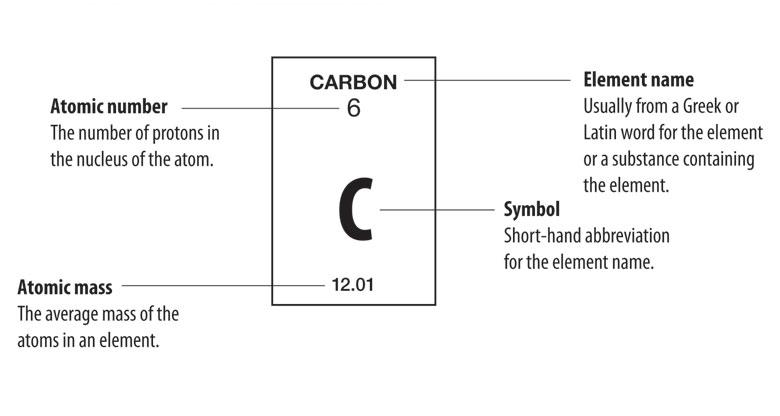
The atomic number (represented by the letter Z)of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular element based solely on its atomic number. For example, any atom with an atomic number of 8 (its nucleus contains 8 protons) is an oxygen atom, and any atom with a different number of protons would be a different element. The periodic table (see figure below) displays all of the known elements and is arranged in order of increasing atomic number. In this table, an element's atomic number is indicated above the elemental symbol. Hydrogen, at the upper left of the table, has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Next on the table is helium, whose atoms have two protons in the nucleus. Lithium atoms have three protons, beryllium atoms have four, and so on.
Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons. In the chemical classroom, the proton count will always be equivalent to an atom's atomic number. This value will not change unless the nucleus decays or is bombarded (nuclear physics).
Mass Number
Experimental data showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number (represented by the letter A)is defined as the total number of protons and neutrons in an atom. Consider the table below, which shows data from the first six elements of the periodic table.
| Name | Symbol | Atomic Number (Z) | Protons | Neutrons | Electrons | Mass Number (A) (rounded to two decimals) |
|---|---|---|---|---|---|---|
| hydrogen | (ce{H}) | 1 | 1 | 0 | 1 | 1.01 |
| helium | (ce{He}) | 2 | 2 | 2 | 2 | 4.00 |
| lithium | (ce{Li}) | 3 | 3 | 4 | 3 | 6.94 |
| beryllium | (ce{Be}) | 4 | 4 | 5 | 4 | 9.01 |
| boron | (ce{B}) | 5 | 5 | 6 | 5 | 10.18 |
| carbon | (ce{C}) | 6 | 6 | 6 | 6 | 12.01 |
Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since (2 + 2 = 4), we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Lithium, for example, has three protons and four neutrons, giving it a mass number of 7.
Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
[text{Number of neutrons} = text{ rounded mass number} - text{atomic number}]
Atoms of the element chromium (left( ce{Cr} right)) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? To determine this, you would subtract as shown:
[52 - 24 = 28 : text{neutrons in a chromium atom}]
The composition of any atom can be illustrated with a shorthand notation called A/Z format. Both the atomic number and mass are written to the left of the chemical symbol. The 'A' value is written as a superscript while the 'Z' value is written as a subscript. For an example of this notation, look to the chromium atom shown below:
[ce{^{52}_{24}Cr}]
Another way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. Symbol-mass format for the above atom would be written as Cr-52. In this notation, the atomic number is not included. You will need to refer to a periodic table for proton values.
Example (PageIndex{1}) Install docker debian.
Calculate each of the three subatomic particles and give specific group or period names for each atom.
- mercury
- platinum
- bromine
Solutions
- Hg (transition metal)- has 80 electrons, 80 protons, and 121 neutrons
- Pt (transition metal)- has 78 electrons, 78 protons, and 117 neutrons
- Br (halogen)- has 35 electrons, 35 protons, and 45 neutrons
Example (PageIndex{2})
Write both A/Z and symbol-mass formats for the atoms in Example (PageIndex{1}).
Solutions
- (ce{^{201}_{80}Hg}) and Hg-201
- (ce{^{195}_{78}Pt}) and Pt-195
- (ce{^{80}_{35}Br}) and Br-80
Example (PageIndex{3})
Identify the elements based on the statements below.
- Which element has 25 protons?
- Which element has 0 neutrons?
- Which element has 83 electrons?
Solutions
a. manganese
b. hydrogen
c. bismuth
Need More Practice?
- Turn to section 3.E of this OER and answer questions #1-#2, #4, and #8.
Contributors and Attributions
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
Chem 1110 - Chapter 2: Atoms, Molecules, and Ions
Practice Quiz 7
QUESTIONS #1-4: Match the discoverers.
1. Nuclear Atom Concept
2. Quantitative relationship between amount of electricity and amount of substance electrolyzed.
3. Charge to mass ratio of electrons in the CRT.
4. Charge of the electron
a) Faradayb) J.J. Thompson
c) Millikan
d) Rutherford
5. How many of the three types of elementary particles are present in a neutral atom of 14C?
The Mass Number Of An Element Is Equal To The

6. A given element is found to contain 90% of isotope having a mass of 19.00 amu and 10% of isotope having a mass of 21.00 amu. What is the atomic weight of the element?
7. Which of the following has a negative charge?
8. Which two subatomic particles have approximately the same mass?
9. What is the atomic weight of a hypothetical element consisting of two isotopes, one with mass = 64.23 amu (26.00%), and one with mass = 65.32 amu?
10. Ernest Rutherford's model of the atom did not specifically include the _____________.
11. The atomic number of an element gives the number of _____________ and _____________ in the atom while the mass number gives the total number of _____________ and _____________.
The Mass Number Of An Element Is Equal To The Number Of Protons And Electrons
12. A hypothetical element consists of the following naturally occurring isotopes. What is the atomic weight of the element?
13. Which of the following has a negative charge?
14. In the Rutherford gold foil experiment, the fact that most of the alpha particles were NOT deflected as they passed through the gold foil indicates that:
15. Which two subatomic particles have approximately the same mass?
16. The number of electrons in a neutral atom of an element is always equal to the _______ of the element.
17. What is the atomic weight of a hypothetical element consisting of two isotopes, one with mass = 64.23 amu (26.00%), and one with mass = 65.32 amu(74.00%)?
18. The difference between the mass number of an atom and the atomic number of the atom is always equal to __________.
19. Isotopes are atoms of the same element that:
20. The atomic number of a certain element is 19, and its atomic weight is 39. An atom of the element contains _____ protons, _____ neutrons, and the chemical symbol for the element is _____.
21. Give the number of protons, neutrons, and electrons in an atom of the 90Sr isotope.
22. Give the number of protons, neutrons, and electrons in the 2141Sc3+ ion.
KEY
1)d 2)a 3)b 4)c 5)b 6)c 7)a 8)e 9)b 10)d 11)d 12)e 13)d 14)b 15)a 16)b 17)d 18)e 19)d 20)c 22)d 22)bTo send electronic mail to Dr. Northrup >snorthrup@tntech.edu, or call 931-372-3421, or come to Foster Hall 407